Alliance of Infinity Trade
Cefdinir
Introducing AIT Pharmaceuticals Division’s Cefdinir Range
At AIT Pharmaceuticals Division, we are dedicated to improving global health through the development and distribution of high-quality pharmaceutical products. Our Cefdinir Range exemplifies our commitment to providing effective antibiotics to healthcare professionals worldwide.
Drug Classification: Cefdinir belongs to the class of antibiotics known as cephalosporins, specifically categorized as a third-generation cephalosporin. This class of antibiotics exhibits broad-spectrum activity against various Gram-positive and Gram-negative bacteria.
Share
Formulations:
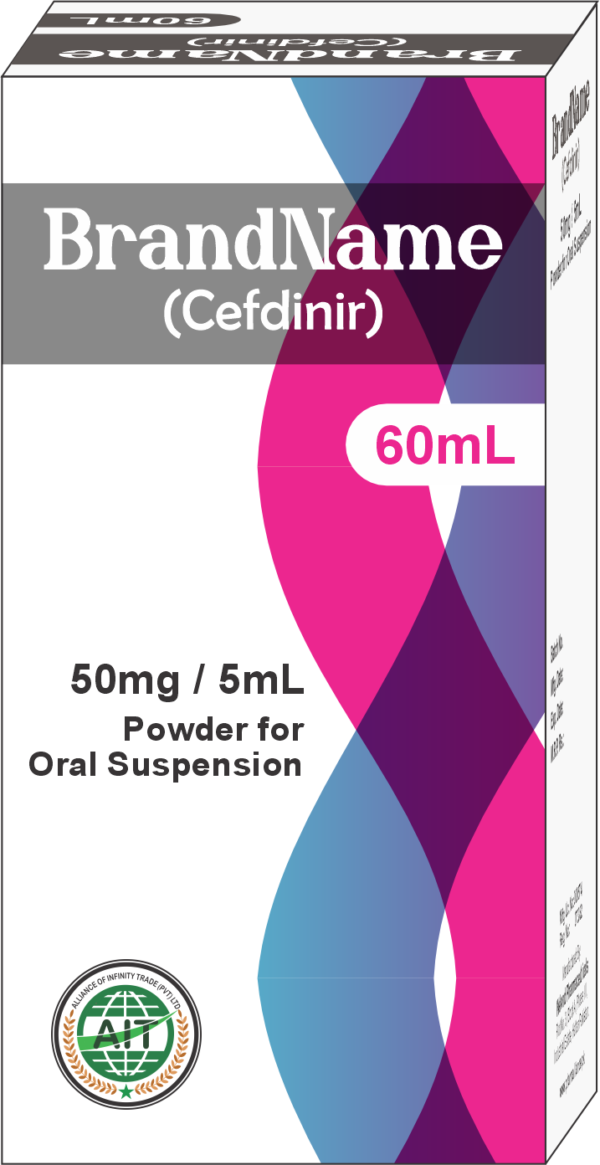
- Cefdinir Dispersible Tablets (DS):
-
-
- Strength: 125mg DS
- Product Form: Dispersible tablets for oral suspension
- Packing Specs: Available in bottles containing 60ml of oral suspension
-
- Cefdinir Capsules:
-
- Strengths: 100mg, 300mg
- Product Form: Hard gelatin capsules for oral administration
- Packing Specs: Available in blister packs of 10 capsules
Indications:
Cefdinir is indicated for the treatment of various bacterial infections caused by susceptible organisms, including:
- Acute exacerbations of chronic bronchitis
- Community-acquired pneumonia
- Acute bacterial sinusitis
- Pharyngitis and tonsillitis
- Uncomplicated skin and skin structure infections
- Acute bacterial otitis media
Pharmacokinetics:
- Absorption: Cefdinir is well absorbed after oral administration, with peak plasma concentrations achieved within 2-4 hours.
- Distribution: The drug distributes widely into various body tissues and fluids, achieving therapeutic concentrations at the site of infection.
- Metabolism: Cefdinir undergoes minimal metabolism in the body, with approximately 20-25% excreted unchanged in the urine.
- Elimination: The elimination half-life of cefdinir is approximately 1.7 hours in adults with normal renal function.
Pharmacodynamics:
- Cefdinir exerts its bactericidal effect by inhibiting bacterial cell wall synthesis through binding to penicillin-binding proteins, leading to cell death.
Usage:
- Cefdinir tablets and dispersible tablets should be taken orally with or without food.
- Dispersible tablets should be reconstituted with water to form a suspension before administration, especially in pediatric patients.
- It is essential to complete the full course of treatment as prescribed by the healthcare provider, even if symptoms improve before the completion of the medication.
Side Effects:
- Common side effects of Cefdinir may include:
- Gastrointestinal disturbances, such as nausea, vomiting, diarrhea, and abdominal pain
- Headache, dizziness, and fatigue
- Skin rash, itching, and urticaria
- Serious side effects such as pseudomembranous colitis and allergic reactions are rare but may occur.
Dosage:
- The dosage of Cefdinir varies depending on the type and severity of the infection, as well as the patient’s age and weight.
- The usual adult dosage for most infections is 300 mg once daily or divided into two doses of 150 mg every 12 hours.
- Pediatric dosages are determined based on the child’s weight and the severity of the infection.
Experience the efficacy and reliability of AIT Pharmaceuticals Division’s Cefdinir Range in the treatment of bacterial infections. For more information, please contact us. We are committed to providing high-quality pharmaceutical products to healthcare professionals worldwide.











Reviews
There are no reviews yet.